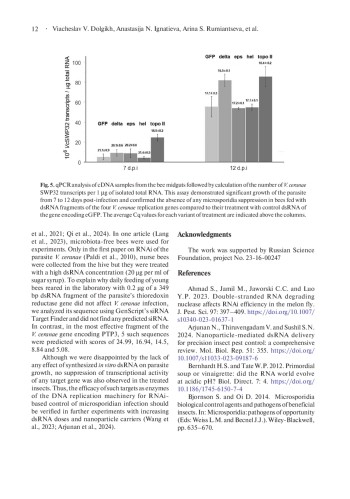
In recent decades, infection of the European honey bee Apis mellifera with the highly virulent microsporidium Vairimorpha (Nosema) ceranae has become globally prevalent. It causes serious losses in beekeeping worldwide and requires new approaches to control bee nosemosis. Since this intracellular parasite has retained components of the RNA interference pathway, double-stranded RNA (dsRNA) treatment of insects may be effective in controlling V. ceranae infection. Inhibition of microsporidia growth in bees fed with 1.8 or even 0.04 μg dsRNA per ml of sugar syrup has been reported in the literature. Considering the crucial role of the genome’s DNA replication machinery for any cell, we synthetized in vitro dsRNA fragments of four V. ceranae genes encoding two subunits (delta and epsilon) of DNA polymerase, helicase and topoisomerase II and fed them to the infected bees with a relatively low dose of 1 µg per ml of sugar syrup. Surprisingly, PCR and qPCR analyses of V. ceranae growth in the midgut of dsRNA-treated insects at 7- and 12-days post-infection revealed neither inhibition of microsporidia growth nor downregulation of target genes. It is worth noting that we collected worker bees of different ages directly from a hive, to simulate the conditions during colony treatment in apiaries. At the same time, newly emerged insects reared in the laboratory were used in the successful experiments mentioned above. This suggests that bee housing conditions as well as gut content may affect the efficiency of RNA interference and requires further increase in dsRNA doses or their mixing with nanoparticle carriers.
Идентификаторы и классификаторы
- SCI
- Биология
Microsporidia are a group of very ancient spore-forming intracellular parasites related to fungi. Almost half of microsporidia genera infect insects, including pests, beneficial parasitoids and predators (Bjørnson and Oi, 2014), as well as domesticated silkworms and honey bees (James and Li, 2012). In the latter case, widespread and destructive infections in bee farms are caused by the microsporidia Vairimorpha (Nosema) ceranae, first discovered in the Asian honey bee Apis cerana (Fries et al., 1996). Although the association of this emergent pathogen with colony losses of honey bees Apis mellifera is still controversial (Higes et al., 2008; Higes et al., 2009; Gisder and Genersch, 2015; Martín-Hernández et al., 2018; Schüler et al., 2023), prevention and treatment of V. ceranae infection in bee hives remains an important task.
Список литературы
1. Ahmad S., Jamil M., Jaworski C.C. and Luo Y.P. 2023. Double-stranded RNA degrading nuclease affects RNAi efficiency in the melon fly. J. Pest. Sci. 97: 397-409. DOI: 10.1007/s10340-023-01637-1
2. Arjunan N., Thiruvengadam V. and Sushil S.N. 2024. Nanoparticle-mediated dsRNA delivery for precision insect pest control: a comprehensive review. Mol. Biol. Rep. 51: 355. DOI: 10.1007/s11033-023-09187-6
3. Bernhardt H.S. and Tate W.P. 2012. Primordial soup or vinaigrette: did the RNA world evolve at acidic pH? Biol. Direct. 7: 4. DOI: 10.1186/1745-6150-7-4
4. Bjornson S. and Oi D. 2014. Microsporidia biological control agents and pathogens of beneficial insects. In: Microsporidia: pathogens of opportunity (Eds: Weiss L.M. and Becnel J.J.). Wiley-Blackwell, pp. 635-670.
5. Cantwell G.E. 1970. Standard methods for counting Nosema Spores. Am. Bee J. 110: 222-223.
6. Desai S.D., Eu Y.J., Whyard S. and Currie R.W. 2012. Reduction in deformed wing virus infection in larval and adult honey bees (Apis mellifera L.) by double-stranded RNA ingestion. Insect. Mol. Biol. 21: 446-455. DOI: 10.1111/j.1365-2583.2012.01150.x
7. Fadeev R.R., Kudryavtseva Yu.S., Bayazyt K-D.K., Shuhalova A.G. and Dolgikh V.V. 2024. The optimized method to isolate dsRNA synthesized in Escherichia coli HT115(DE3). Agricultural Biology. 59: 460-472. DOI: 10.15389/agrobiology.2024.3.460eng
8. Fries I., Feng F., da Silva A., Slemenda S.B. and Pieniazek N.J. 1996. Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur. J. Protistol. 32: 356-365.
9. Gisder S. and Genersch E. 2015. Identification of candidate agents active against N. ceranae infection in honey bees: establishment of a medium throughput screening assay based on N. ceranae infected cultured cells. PLoS One. 10: e0117200. DOI: 10.1371/journal.pone.0117200
10. Guan R., Chu D., Han X., Miao X. and Li H. 2021. Advances in the development of microbial double-stranded RNA production systems for application of RNA interference in agricultural Pest Control. Review Front. Bioeng. Biotechnol. 9: 753790. DOI: 10.3389/fbioe.2021.753790
11. Guan R.B, Li H.C., Fan Y.J., Hu S.R. et al. 2018. A nuclease specific to lepidopteran insects suppresses RNAi. J. Biol. Chem. 293: 6011-6021. DOI: 10.1074/jbc.RA117.001553
12. He N., Zhang Y., Duan X.L., Li J.H. et al. 2021. RNA interference-mediated knockdown of genes encoding spore wall proteins confers protection against Nosema ceranae infection in the European honey bee, Apis mellifera. Microorganisms. 9: 505. DOI: 10.3390/microorganisms9030505 EDN: CYZPFJ
13. Higes M., Martín-Hernández R., Botías C., Bailón E.G., González-Porto A.V. et al. 2009. Honeybee colony collapse due to Nosema ceranae in professional apiaries. Environ. Microbiol. Rep. 1: 110-113. DOI: 10.1111/j.1758-2229.2009.00014.x
14. Higes M., Martín-Hernández R., Garrido- Bailón E. et al. 2008. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 10: 2659-2669. DOI: 10.1111/j.1462-2920.2008.01687.x
15. James R.R. and Li Z. 2012. From silkworms to bees: diseases of beneficial Insects. In: Insect Pathology (Eds: Vega F.E. and Kaya H.K.). Elsevier Inc., pp. 429-459.
16. Ignatieva A.N., Timofeev S.A., Tokarev Y.S. and Dolgikh V.V. 2022. Laboratory cultivation of Vairimorpha (Nosema) ceranae (Microsporidia: Nosematidae) in artificially infected worker bees. Insects. 13: 1092. DOI: 10.3390/insects13121092
17. Lang H.Y., Wang H., Wang H.Q., Zhong Z.P. et al. 2023. Engineered symbiotic bacteria interfering redox system inhibit microsporidia parasitism in honeybees. Nat.Commun. 14: 2778. DOI: 10.1038/s41467-023-38498-2
18. Lin-Ling W., Ke-Ping C., Ze Z., Qin Y. et al. 2006. Phylogenetic analysis of Nosema antheraeae (Microsporidia) isolated from Chinese oak silkworm, Antheraea pernyi. J. Eukaryot. Microbiol. 53: 310-313. DOI: 10.1111/j.1550-7408.2006.00106.x
19. Maori E., Paldi N., Shafir S., Kalev H. et al. 2009. IAPV, a bee-affecting virus associated with colony collapse disorder can be silenced by dsRNA ingestion. Insect. Mol. Biol. 18: 55-60. DOI: 10.1111/j.1365-2583.2009.00847.x
20. McGruddy R.A., Smeele Z.E., Manley B., Masucci J.D. et al. 2024. RNA interference as a next-generation control method for suppressing Varroa destructor reproduction in honey bee (Apis mellifera) hives. Pest. Manag. Sci. 80: 4770-4778. DOI: 10.1002/ps.8193
21. Martín-Hernández R., Bartolomé C., Chejanovsky N., Le Conte Y. et al. 2018. Nosema ceranae in Apis mellifera: a 12 years postdetection perspective. Environ. Microbiol. 20: 1302-1329. DOI: 10.1111/1462-2920.14103
22. Ndikumana S., Pelin A., Williot A., Sanders J. L. et al. 2017. Genome analysis of Pseudoloma neurophilia: a microsporidian parasite of zebrafish (Danio rerio). J. Eukaryot. Microbiol. 64: 18-30. DOI: 10.1111/jeu.12331
23. Paldi N., Glick E., Oliva M., Zilberberg Y. et al. 2010. Effective gene silencing in a microsporidian parasite associated with honeybee (Apis mellifera) colony declines. Appl. Environ. Microbiol. 76: 5960- 5964. DOI: 10.1128/AEM.01067-10
24. Pereira T.C. and Lopes-Cendes I. 2013. Medical applications of RNA interference (RNAi). BMC Proc. 7: K21. DOI: 10.1186/1753-6561-7-S2-K21
25. Qi Y., Wang C., Lang H., Wang Y. et al. 2024. Liposome-based RNAi delivery in honeybee for inhibiting parasite Nosema ceranae. Synth. Syst. Biotechnol. 9: 853-860. DOI: 10.1016/j.synbio.2024.07.003
26. Rodríguez-García C., Evans J.D., Li W., Branchiccela B. et al. 2018. Nosemosis control in European honey bees, Apis mellifera, by silencing the gene encoding Nosema ceranae polar tube protein 3. J. Exp. Biol. 221: jeb184606. DOI: 10.1242/jeb.184606
27. Rodrigues T.B., Mishra S.K., Sridharan K., Barnes E.R. et al. 2021. First sprayable double-stranded RNA-based biopesticide product targets proteasome subunit beta type-5 in Colorado potato beetle (Leptinotarsa decemlineata). Front. Plant. Sci. 12: 728652. DOI: 10.3389/fpls.2021.728652
28. Romeis J. and Widmer F. 2020. Assessing the risks of topically applied dsRNA-based products to non-target arthropods. Front. Plant Sci. 11: 679. DOI: 10.3389/fpls.2020.00679
29. Schüler V., Liu Y.C., Gisder S., Horchler L. et al. 2023. Significant, but not biologically relevant: Nosema ceranae infections and winter losses of honey bee colonies.Commun. Biol. 6: 229. DOI: 10.1038/s42003-023-04587-7
30. Timofeev S., Tsarev A. Senderskiy I., Rogozhin E. et al. 2019. Efficient transformation of the entomopathogenic fungus Lecanicillium muscarium by electroporation of germinated conidia. Mycoscience. 60: 197-200. DOI: 10.1016/j.myc.2019.02.010
31. Tokarev Y.S., Timofeev S.A., Malysh J.M., Tsarev A.A. et al. 2018. Hexokinase as a versatile molecular genetic marker for microsporidia. Parasitology. 15: 1-7. DOI: 10.1017/S0031182018001737
32. Wang Y., Yan Q., Lan C., Tang T. et al. 2023. Nanoparticle carriers enhance RNA stability and uptake efficiency and prolong the protection against Rhizoctonia solani. Phytopathology Research. 5: 2. DOI: 10.1186/s42483-023-00157-1
33. Zheng H., Powell J.E., Steele M.I., Dietrich C. and Moran N.A. 2017. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc. Natl. Acad. Sci. USA. 114: 4775-4780.
Выпуск
Другие статьи выпуска
Thecamoeba onigiri n. sp. (Amoebozoa, Discosea, Thecamoebida) was isolated from a moss sample collected in the surroundings of Lake Baikal (Russia). The amoebae of this species belong to the striate morphotype and have a single rounded nucleus. The nucleolar material of T. onigiri can be organized in different ways depending on the age of the culture. The most common was a nucleus with a nearly spherical eccentric nucleolus, sometimes located very close to the nuclear envelope. The surface of such a nucleolus was rough and uneven. This type of nucleolar organization was observed in one- to two-week-old cultures. In contrast, a centrally located rounded nucleolus with smooth surface predominated in three- to four-week-old cultures. This type of the nucleolus corresponds to the classical “vesicular” nucleus and is similar to that found in amoebae of “T. quadrilineata species group”, but differs in having lacunae that are more peripheral. Molecular phylogenetic analysis based on 18S rRNA gene sequences showed that T. onigiri forms a clade with T. astrologa as part of a larger group that also includes the “T. quadrilineata species group” and T. aesculea. Meanwhile, two species of Thecamoeba demonstrate polymorphism of the nucleolar material arrangement, and both these species are phylogenetically close.
Protists inhabit waters with different salinities up to saturation, and play significant roles in the food webs. In hypersaline environments, the simplified communities are composed of mainly pro- and eukaryotic halophilic microorganisms. The aim of this study was to characterize the prokaryotic assemblages associated with uniprotist cultures of heterotrophic and phototrophic halophilic protists isolated from inland saline water bodies and maintained under laboratory conditions for a long time. The cultures were represented by chlorophycean algae Dunaliella and Asteromonas, as well as by heterotrophic protozoa of the phylum Heterolobosea. DNA metabarcoding revealed that the prokaryotic assemblages differed drastically between the phototrophic and heterotrophic protists in richness, diversity and taxonomic composition. Generally, the prokaryotic assemblages of Heterolobosea protozoa were richer than those of chlorophycean algae. In the algal microbiomes, only few prokaryotic genera were revealed. They were represented by the bacterial phylum Pseudomonadota and the archaeal phylum Halobacteriota. Rather diverse prokaryotic assemblages were associated with the cultures of halophilic Heterolobosea protozoa. They were composed of the phyla Pseudomonadota, Bacteroidota, Balneolota, Thermodesulfobacteriota, Halobacteriota, and Nanohaloarchaeota. Predicted functional profiles of the prokaryotic assemblages revealed the pathways responsible for close metabolic interactions between the halophilic protists and their prokaryotic associates, including synthesis of organic osmotic solutes and their conversion, degradation of sugar and organic aromatic compounds, biosynthesis of cofactors and vitamins. The dramatic differences between prokaryotic assemblages of heterotrophic and phototrophic protists suggest the existence of different selection mechanisms shaping microbiomes of the halophilic protists that still have to be elucidated.
Although the global distribution of eumycetozoans in terrestrial ecosystems with varying vegetation types has been a subject of a number of investigations during the past decade, there is still scarce to no available data from the mangrove forests, particularly in the Philippines. Hence, this study assesses and compares the occurrence and distribution of protosteloid amoebae inhabiting the mangrove ecosystem of San Fernando City, La Union, with the villages Biday and Catbangen as representatives. Aerial (AL) and ground (GL) leaf litter samples were used as substrates in isolating protosteloid amoebae and were subjected to moist chamber cultures. The 17 species belonging to 12 genera, from a total of 125 records, were described and reported in this study, with Protostelium mycophagum being the most often occurring species. Further results indicate that the ground microhabitat and the Biday site exhibited higher species diversity and abundance than the aerial microhabitat and the Catbangen site. Regarding species richness in the two leaf litters, GL hosted higher species richness than AL. The current research is one of the few that has assessed and surveyed the eumycetozoan distribution, occurrence, and ecology in the Philippine mangrove ecosystem. Furthermore, it demonstrates the potential for a mangrove forest to support diverse protosteloid amoebae growth.
The PII superfamily consists of widespread signal transduction proteins found in all domains of life. The most conserved PII-interactor across oxygenic phototrophs from cyanobacteria to Archaeplastida is the key enzyme of the ornithine/arginine synthesis pathway, N-acetyl-L-glutamate kinase (NAGK). T-loops represent the major PII-receptor binding element and are involved in the interaction with NAGK. Within the class Mamiellophyceae, only the genus Micromonas contains species with the PII protein. Bioinformatic analysis revealed that the PII protein of Micromonas pusilla (MpPII) has an unusually prolonged T-loop. Here, we performed the coupled enzyme assay and showed that MpPII has no remarkable influence on NAGK activity. An engineered variant of MpPII with deletion of four additional amino acids (AATD) in the T-loop restored the ability of this protein to relieve NAGK from feedback inhibition by arginine in a glutamine-dependent manner. The findings are discussed in the context of unusual plasticity of the PII protein family during the evolution of Archaeplastida.
Статистика статьи
Статистика просмотров за 2025 год.
Издательство
- Издательство
- ИНЦ
- Регион
- Россия, Санкт-Петербург
- Почтовый адрес
- Санкт-Петербург, 194064, Тихорецкий проспект, 4
- Юр. адрес
- Санкт-Петербург, 194064, Тихорецкий проспект, 4
- ФИО
- Томилин Алексей Николаевич (Руководитель)
- E-mail адрес
- cellbio@incras.ru
- Контактный телефон
- +7 (812) 2971878
- Сайт
- https:/incras.ru